electronic configuration cu|cu+2 electron configuration : Cebu Chlorine (Cl) - Electron Configuration for Copper (Cu, Cu+, Cu2+) - UMD
I know it becomes obvious by the end, but I love the sprinkling of hints that Daphne's loving the experience: She willingly bounces on the mummy's dick, she takes the 69-ing ghost's cock down her throat and then swallows (which must feel amazing on his glans--hence his thumbs up), her deep-throating the vampire so hard that he tenses up .
electronic configuration cu,The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. How to Write .In order to write the Calcium electron configuration we first need to know the .electronic configuration cuIn order to write the Mg electron configuration we first need to know the .
electronic configuration cu cu+2 electron configurationIn order to write the Mg electron configuration we first need to know the .
When we write the configuration we'll put all 19 electrons in orbitals around the .In order to write the Silicon electron configuration we first need to know the .
Chlorine (Cl) - Electron Configuration for Copper (Cu, Cu+, Cu2+) - UMDLithium is the third element with a total of 3 electrons. In writing the electron .Hydrogen (H) - Electron Configuration for Copper (Cu, Cu+, Cu2+) - UMDFluorine - Electron Configuration for Copper (Cu, Cu+, Cu2+) - UMD
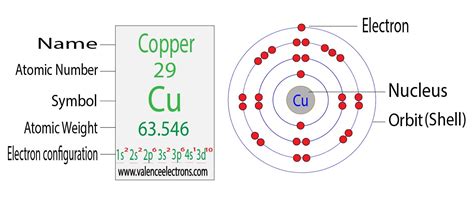
The electron configuration of copper (Cu) includes a fully-filled 3d subshell. Cu: 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. The actual electron configuration of . Electronic Configuration of Cu. The electronic configuration of copper (Cu) can be represented as: 1s2 2s2 2p6 3s2 3p6 4s1 3d10. This configuration indicates that . Causey shows you step by step how to write the electron configuration and orbital notation for copper (Cu). You will learn about the orbital overlapping and the electron jump that occurs is in.

1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of Cu. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9.
Jan 25, 2014. Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table. This would make the electron configuration for . The electronic configuration of copper (Cu), with an atomic number of 29, is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. This unique configuration is characterized by one electron .
Copper, a transition metal with the symbol Cu, and atomic number 29, is a d-block element in the periodic table. Let us discuss the electronic configuration of Cu. The .

In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the .
From Sc on, the 3 d orbitals are actually lower in energy than the 4 s orbital, which means that electrons enter the 3 d orbitals first. In this video, we’ll discuss this in more depth and .Solution. The correct option is B 1s2,2s2p6,3s2p6d10,4s1. Cu:1s2,2s2p6,3s23p63d10,4s1. In case of copper, a completely full or half full d sub-level is more stable than a partially filled d sub-level, so an electron from the 4s orbital is .
The Electron: Crash Course Chemistry #5. Video 2.6.2 2.6. 2: An overview of the role of orbitals in electron configurations and how to write electron configurations. The relative energy of the subshells determine the order in which atomic orbitals are filled (1 s, 2 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, and so on). The electronic configuration of copper (Cu), with an atomic number of 29, is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. This unique configuration is characterized by one electron in the 4s orbital and ten electrons in the 3d orbital, which differs from the typical filling order. Copper’s 3d¹⁰ configuration in the third energy level (shell .
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Copper (Cu) [Ar] 3d 10 4s 1: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1: 2, 8, 18, 1: 30: Electron configuration of Zinc (Zn) [Ar] 3d 10 4s 2: 1s 2 2s 2 2p 6 3s 2 . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number . Introduction to electron configurations. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of . Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table.This would make the electron configuration for copper, #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^9# or in noble gas configuration [Ar] #4s^2 3d^9#.. However, because the 3d orbital is so much larger then the 4s orbital and the 3d . Electronic Structure. The electronic configuration of transition metal elements are characterized as having full outer sub-orbitals and the second outermost d sub-orbitals incompletely filled, with the exception of Copper which loses one 4s orbital electron to the 3d sub-orbital for increased stability. The electron configuration for .
Configuration électronique du cuivre. La configuration électronique du cuivre est 1s2 2s2 2p6 3s2 3p6 4s2 3d9. Le cuivre est un métal de transition qui fait partie de la famille du cuivre avec l'or et l'argent. Il a une couleur cuivrée .Electronic configuration of Copper: The atomic number of Copper(Cu) = 29; Therefore, the expected electronic configuration is Ar 3 d 9 4 s 2. But Copper has an exception in electronic configuration due to the stability concept of orbitals, completely filled and half filled orbitals are the most stable. Thus, the observed electronic of copper is .
Let us use this smart electron configuration calculator to determine the electron configuration of copper: Choose copper (Cu) from the drop-down list of elements. Check the atomic number (29) and atomic mass (63.546) of copper. Read here how to calculate atomic mass. Read the full electron configuration:
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .
cu+2 electron configurationThis explains the anomalous electron configuration of the transition metals and allows us to refine the electron configuration of Cu as: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 10 (paramagnetic, 1 unpaired electron) and so becomes Cu +: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 (diamagnetic; no unpaired electrons) so that we are consistent with experimental data. For example, silicon has nine possible integer oxidation states from −4 to +4, but only -4, 0 and +4 are common oxidation states. Copper - Electron Configuration and Oxidation States - Cu. Electron configuration of Copper is [Ar] 3d10 4s1. Possible oxidation states are +1,2.
The electron configuration of an atom describes how the electrons are arranged in the atom’s energy levels, subshells, and orbitals. For a neutral atom of beryllium (Be), which has an atomic number of 4, the electron configuration is written as 1s²2s². This means that the first energy level (shell) has 1s², indicating two electrons in.
The electronic configuration of Cu is [Ar]4s 1 3d 10 . When the water molecules are sharing 8 electrons, it becomes 4s2 3d10 4p6 5s1. When cu becomes cu2+ion, the configuration becomes 4s2 3d10 4p5. But these 2 configurations aren't stable. Then why did cuso4.5h2o occurs at a reasonably good amount in nature.
electronic configuration cu|cu+2 electron configuration
PH0 · sc electronic configuration
PH1 · magnesium electronic configuration
PH2 · fe electronic configuration
PH3 · electronic configuration of carbon
PH4 · electronic configuration of argon
PH5 · cu+2 electron configuration
PH6 · configuration of copper
PH7 · co electronic configuration
PH8 · Iba pa